Boston Scientific's Farapulse Is a Showstopper
Electrophysiologists are giving the new pulsed field ablation system a warm welcome.
April 25, 2024
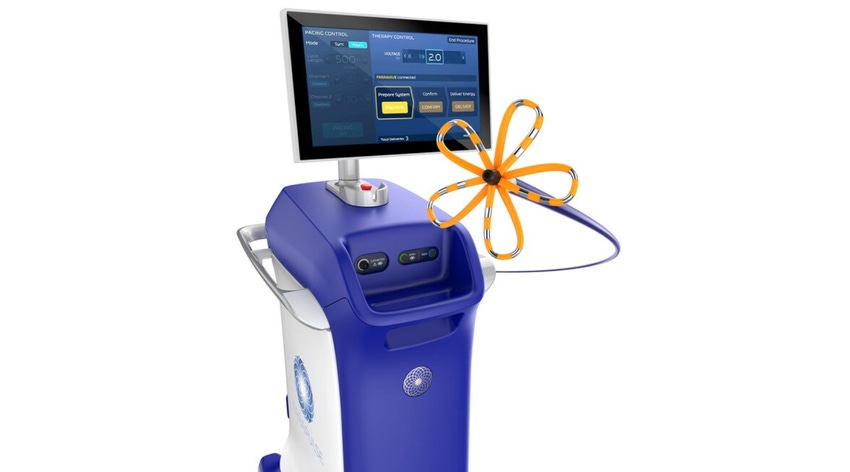
Boston Scientific CEO Mike Mahoney says Farapulse, the company’s pulsed field ablation (PFA) system, is the most transformational product he has seen in his 12-year career with the company. But don’t just take his word for it; one look at the company’s first-quarter earnings solidly backs up the sentiment.
FDA approved Farapulse in January, and the launch powered 85% growth in Boston Scientific’s U.S. electrophysiology business in the first quarter, compared to the same quarter last year.
“We continue to invest significantly in the current platform and in future products and a significant amount in clinical science to really widen the gap in PFA with our Farapulse system,” Mahoney said. “...It’s a remarkable platform, and we’re seeing excellent safety results, ease of use, rapid adoption, and excellent effectiveness.”
PFA has been touted as a promising new ablation modality for the treatment of atrial fibrillation. Boston Scientific began investing in Farapulse in 2014, and signaled its intent to buy Farapulse in 2020, following through on that intent in 2021. Farapulse was first-to-market in Europe and second-to-market in the United States, after Medtronic. FDA approved Medtronic’s PulseSelect PFA system late last year.
During a traditional ablation procedure, a catheter is guided to the interior of the heart and generates extreme temperatures – hot or cold – to destroy targeted areas associated with abnormal heart rhythms. The Farapulse PFA System, however, relies on tissue-selective, non-thermal electric fields to ablate heart tissue and avoid damage to surrounding structures.
Johnson & Johnson is also developing a PFA system, along with private medtech companies Kardium, Galvanize, and Adagio. But Ken Stein, MD, Boston Scientific’s chief medical officer, cautions spectators against thinking of PFA in general because each of these technologies have significant differences in design.
“The results you get with PFA are just completely dependent on catheter design, on waveform, and on dosing strategy,” Stein said. “When people see the data – and we’ve now got published data, including randomized clinical trial data, registry data, total data, well over 18,000 patients have been reported at this point – it just has compelling advantages in terms of safety, in terms of efficacy, in terms of ease of use, and in terms of efficiency. And that’s what you see driving the rapid uptake, both in the U.S. and globally.”
Mahoney said the company is also seeing multiple hospitals buying a second console, which speaks to the adoption and frequency of use.
The current version of Farapulse requires physicians to use separate mapping and ablation catheters. Boston Scientific is working to add a next-generation Farawave catheter with navigation capabilities to its roster. That device could be on the U.S. market by late 2024, the company noted during an investor event last year.
“It is our desire and our belief that there will be people who don't feel a need to use any mapping when they're doing pulmonary vein isolation with Farapulse, who are adopting a very efficient workflow that's been used in a lot of centers in Europe. There will be others who want to continue to use a navigation mapping system,” Stein said. “We have no intention of forcing people to use our system. We're not going to lock it down.”
But, he noted, the next-generation system will offer some compelling advantages for the physicians who want to use the navigation mapping capabilities.
“As Dr. Stein mentioned, we really want to have hospitals choose the correct mapping system, and Farapulse works excellent today with competitors, but we expect some enhanced benefit with ours,” Mahoney said.
The company declined to share specific Farapulse numbers this early in the game, although one analyst on the call estimated that Farapulse revenue was somewhere around $40 million between the generator and the catheter itself.
“We won’t confirm your math,” CFO Dan Brennan told the analyst. “...But it’s going extremely well. And importantly, we made significant investments in the supply chain two and three years ago to be ready for where we are today.”
You May Also Like


